This page/content is for Great Britain healthcare professionals only.
Pluvicto® is indicated for the treatment of adult patients with prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC) who have been treated with androgen receptor (AR) pathway inhibition and taxane-based chemotherapy or who are not medically suitable for taxanes.2
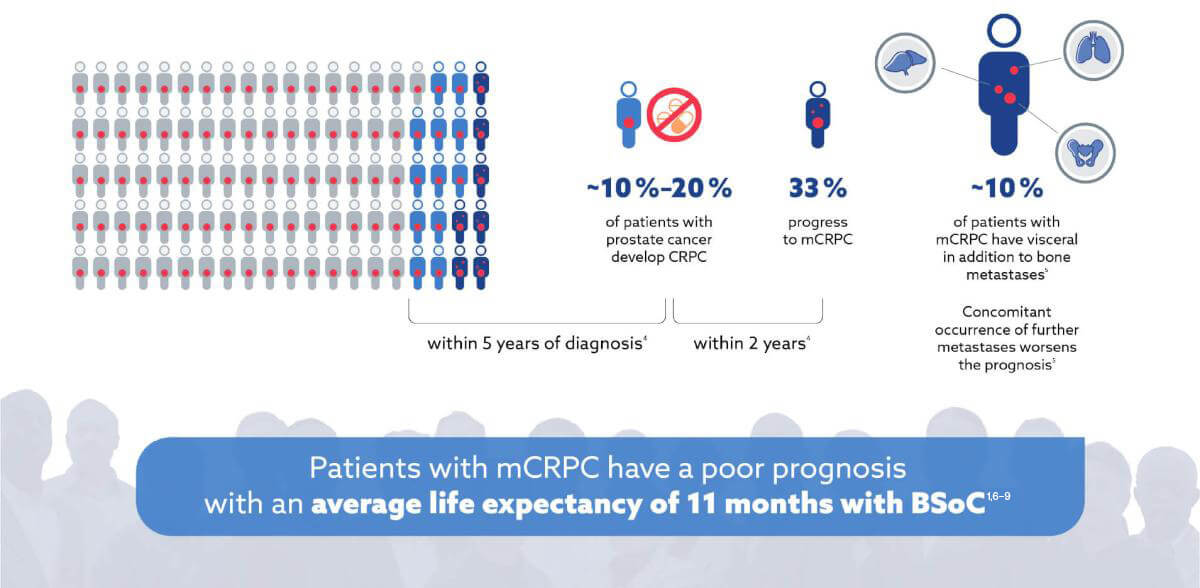
mCRPC and PSMA
When patients progress to mCRPC their prognosis decreases significantly3,4
PSMA scanning enables you to see and target PSMA-positive mCRPC12–14
![]()
The high sensitivity of PSMA scanning detects PSMA-positive bone, nodal, or visceral metastases13–16
![]()
PSMA imaging establishes patient eligibility for PSMA-targeted RLT1
The most common ADRs (≥20%) occurring at a higher incidence in patients who received Pluvicto + BSoC compared to BSoC alone include: fatigue, dry mouth, nausea, anaemia, decreased appetite and constipation. For the full list of ADRs, please refer to the Pluvicto® Summary of Product Characteristics.
Prescribing information for Locametz®▼ (gozetotide) and adverse event reporting details can be found here.
*In VISION, a randomised, open-label, multicentre, Phase III clinical trial (n=831), Pluvicto prolonged overall survival, imaging-based progression-free survival, and maintained quality of life for longer when used alongside best standard of care compared to best standard of care alone.
†Alternate primary endpoints: Overall survival was improved by 4 months vs BSoC (HR 0.62, 95% CI, 0.52–0.74; p<0.001), radiographic progression-free survival was improved by 5.3 months vs BSoC (HR 0.40, 99.2% CI, 0.29–0.57; p<0.001).
‡Secondary endpoint: Quality of life measured as FACT-P total score was maintained by 3.5 months more vs BSoC (HR 0.54, 95% CI, 0.45–0.66; nominal p-value with non-inferential analysis <0.001), quality of life measured as BPI-SF pain intensity was maintained by 3.7 months more vs BSoC (HR 0.52, 95% CI, 0.43–0.63, nominal p-value with non-inferential analysis <0.001).
ADR, adverse drug reaction; AR, androgen receptor; BPI-SF, brief pain inventory (short form); BSoC, best standard of care; CI, confidence interval; FACT-P, functional assessment of cancer therapy – prostate; HR, hazard ratio; mCRPC, metastatic castration-resistant prostate cancer; PSMA, prostate-specific membrane antigen; RLT, radioligand therapy.
References:
- Sartor O, et al. N Engl J Med 2021;385(12):1091–1103.
- Pluvicto® Great Britain Summary of Product Characteristics. Available at: https://www.medicines.org.uk/emc/product/13965/smpc [Accessed March 2023].
- Kyriakopoulos CE, et al J Clin Oncol. 2018;36(11):1080–1087.
- Kirby M, et al. Int J Clin Pract 2011;65(11):1180–1192.
- Zhao F, et al. Clin Transl Med 2019;8(1):30.
- Fizazi K, et al. Lancet Oncol 2012;13(10):983–992. Erratum in: Lancet Oncol 2012;13(11):e464. Erratum in: Lancet Oncol. 2014;15(9):e365.
- Scher HI, et al. N Engl J Med 2012;367(13):1187–1197.
- Hoskin P, et al. Lancet Oncol 2014;15(12):1397–1406.
- de Wit R, et al. N Engl J Med 2019;381(26):2506–2518.
- Sartor O, et al. N Engl J Med 2021;385(12):1091–1103. Supplementary appendix.
- Pomykala KL et al, J Nucl Med 2020;61(3):405–411.
- Hofman MS, et al. Lancet Oncol 2018;19(6):825–833.
- Maffey-Steffan J, et al. Eur J Nucl Med Mol Imaging 2020;47(3):695–712. Erratum in: Eur J Nucl Med Mol Imaging 2019; PMID: 31776632; PMCID: PMC7005064.
- Vlachostergios PJ, et al. Front Oncol 2021;11:630589.
- Woythal N, et al. J Nucl Med 2018;59(2):238–243.
- Schmuck S, et al. J Nucl Med 2017;58(12):1962–1968.

