Pluvicto®▼ (lutetium [177Lu] vipivotide tetraxetan) is indicated for the treatment of adult patients with prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC) who have been treated with androgen receptor (AR) pathway inhibition and taxane-based chemotherapy or who are not medically suitable for taxanes.1
Dosing and administration
Please refer to the Pluvicto Summary of Product Characteristics for full information about dosing and administration.1
Pluvicto is a ready-to-use solution. Be aware that treatment with Pluvicto contributes to a patient's overall long-term cumulative radiation exposure, which is associated with an increased risk for cancer.1
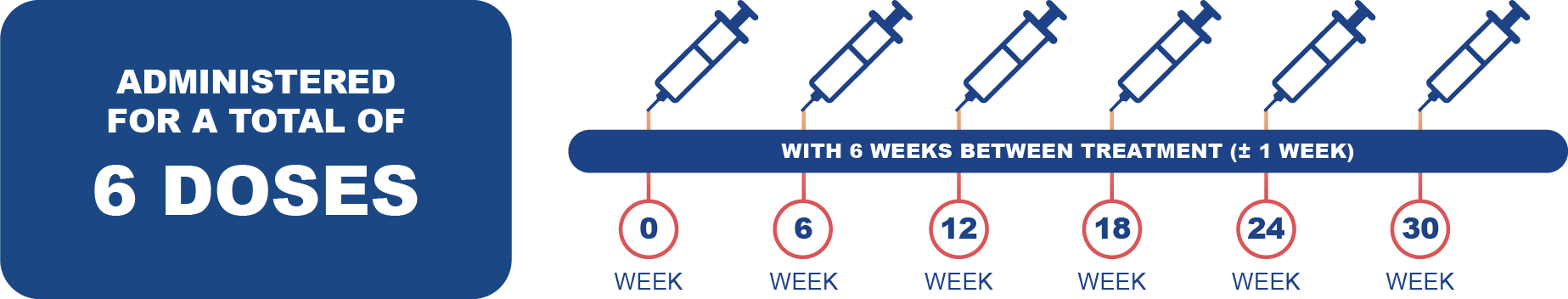
Posology: Pluvicto is administered every 6 weeks (± 1 week) for a total of 6 doses.1
One dose of Pluvicto contains 7.4 GBq (~200 mCi) – at time of use.1
Be aware that treatment with Pluvicto contributes to a patient's overall long-term cumulative radiation exposure, which is associated with an increased risk for cancer. Management of severe or intolerable ADRs may require temporary dose interruption (extending the dosing interval from 6 weeks up to 10 weeks), dose reduction, or permanent discontinuation of treatment with Pluvicto. Please refer to the Pluvicto SmPC for further information regarding dose reductions.
There are 3 ways to administer Pluvicto:1
- As an injection using a disposable syringe fitted with a syringe shield (with or without a syringe pump)
- As an infusion using the gravity method (with or without an infusion pump)
- As an infusion using the vial (using a peristaltic infusion pump)
For more information on the 3 administration methods, please watch the videos below.
Pluvicto®▼ (lutetium [177Lu] vipivotide tetraxetan) Prescribing Information
Pluvicto should be administered only by persons authorised to handle radiopharmaceuticals in designated clinical settings and after evaluation of the patient by a qualified physician.
Radiopharmaceuticals, including Pluvicto, should be used by or under the control of healthcare professionals who are qualified by specific training and experience in the appropriate use and handling of radiopharmaceuticals, and whose experience and training have been approved by the appropriate governmental agency authorised to license the use of radiopharmaceuticals.
Pluvicto is a radiopharmaceutical and should be handled with appropriate safety measures to minimise radiation exposure. Waterproof gloves and effective radiation shielding should be used when handling Pluvicto. Radiation exposure to patients, medical personnel, and household contacts should be minimised during and after treatment with Pluvicto consistent with institutional good radiation safety practices, patient management procedures, and instructions to the patient for follow-up radiation protection at home.
The most common adverse reactions (≥20%) occurring at a higher incidence in patients who received Pluvicto + BSoC were fatigue (43.1%), dry mouth (39.3%), nausea (35.3%), anaemia (31.8%), decreased appetite (21.2%) and constipation (20.2%).1 Clinically relevant adverse reactions in <5% of patients included dry eye, vertigo and pancytopenia (including bicytopenia).1
For further information please refer to the Pluvicto SmPC.
ADR, adverse drug reaction; AR, androgen receptor; BSoC, best standard of care; mCRPC, metastatic castration-resistant prostate cancer; PSMA, prostate-specific membrane antigen; SmPC, summary of product characteristics.
Reference:
- Pluvicto®▼ (lutetium [177Lu] vipivotide tetraxetan) Summary of Product Characteristics.

